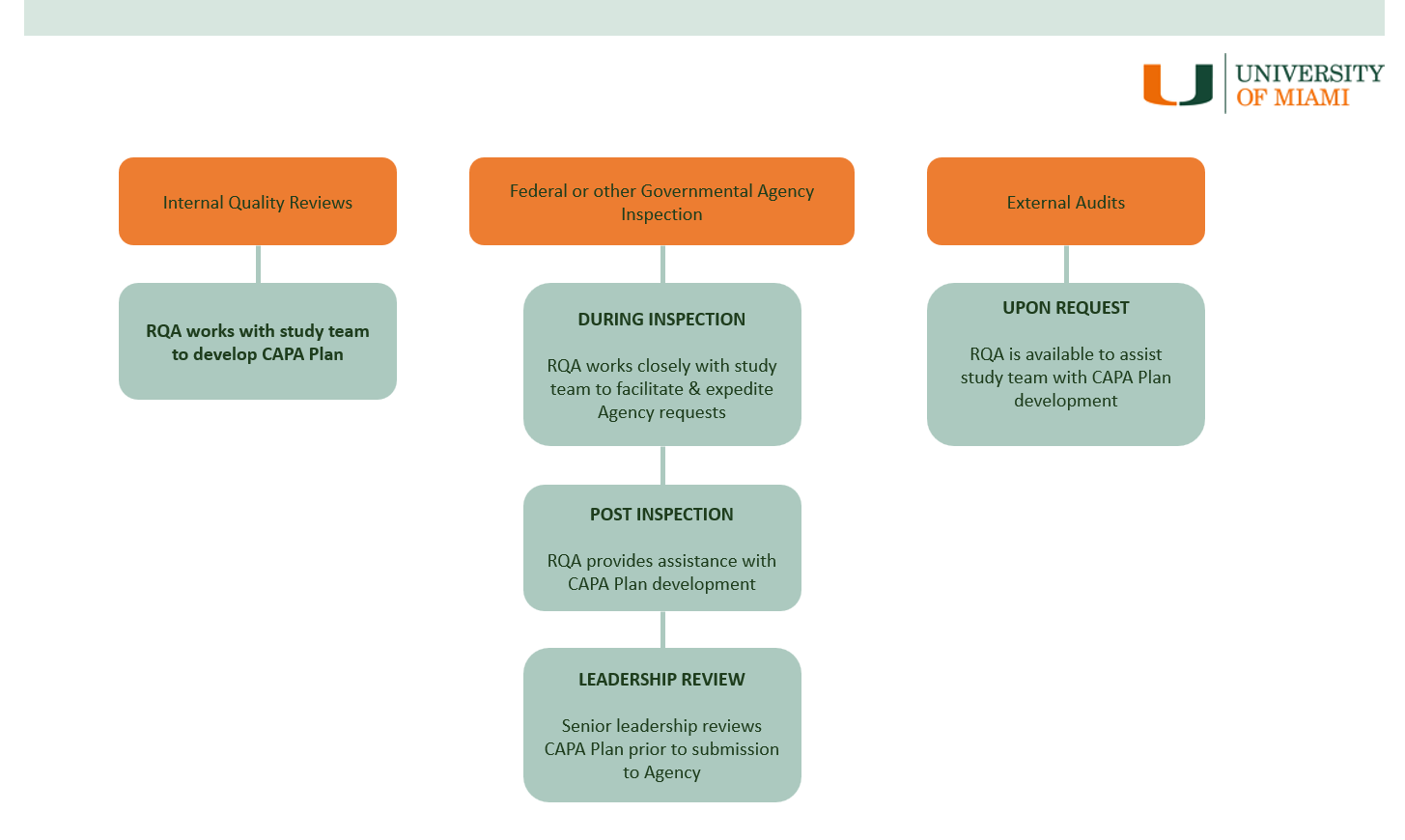
During an external audit conducted by a federal or other governmental agency, RQA works closely with the PI and study team to facilitate and expedite the process of responding to questions or requests from the federal/governmental inspector(s).
At the conclusion of such audit, a CAPA Plan may be requested by federal agencies such as FDA, OHRP, NIH, DOD, EMA.
RQA advises on strategies for corrective/preventive measures and assists in the development and execution of a CAPA Plan that addresses all observations, findings, or concerns noted by the inspecting agency.
🔗 Please reference Policy: Corrective Action Preventive Action (CAPA) Plans in Human Subject Research